MRsafe – Prototypical Development and Evaluation of a Mobile Application for Healthcare Professionals to Query the MR Compatibility of Cardiac Implantable Electronic Devices
Aim and Research Question(s)
Development and evaluation of a prototypical app to help healthcare professionals find the necessary MRI safety information on cardiac implantable electronic devices (CIEDs), so that MRI examinations can be performed safely on patients with CIEDs. RQ 1: What kind of information from cardiac implantable electronic devices is necessary to ensure a safe MRI examination? RQ 2: What are the functional and technical requirements for the mobile application to support healthcare professionals in determining the MR compatibility of cardiac implantable electronic devices? RQ 3: What are the task completion rate and task time as usability metrics when testing a prototypical service for assessing the MR compatibility of cardiac implantable electronic devices?
Background
Patients with CIEDs still report difficulties in accessing MRI examinations worldwide, despite the first MR conditional pacemaker system becoming available in 2008 (EU) and 2011 (US). Reasons for this include safety concerns about patient risk [1], [2].
Methods
The method used for the prototypical development and evaluation was a user-centered design (UCD) process, a mixed method with qualitative and quantitative approaches (Figure 1).
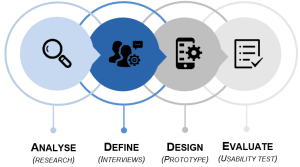 Figure 1: User-centered design method
Figure 1: User-centered design method
Results and Discussion
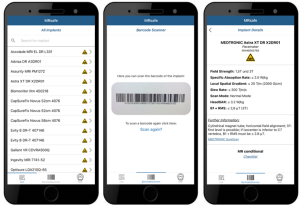 Figure 2: Screenshots of the prototypical MRsafe app
Figure 2: Screenshots of the prototypical MRsafe app
 Table 1: Task completion rate results from the usability test
Table 1: Task completion rate results from the usability test
 Table 2: Task time results from the usability test
Table 2: Task time results from the usability test
Conclusion
The results of this master thesis suggest that the use of an app to query the MR compatibility of CIEDs can be a good alternative to already existing sources, as an improvement in both task completion rate and task time was achieved. Further development of the prototype as well as a larger study could be aimed at.
References
[1] C. Pieri et al., ‘Access to MRI for patients with cardiac pacemakers and implantable cardioverter defibrillators’, Open Heart, vol. 8, no. 1, pp. 1–6, May 2021, doi: 10.1136/openhrt-2021-001598. [2] W. Korutz et al., ‘Pacemakers in MRI for the Neuroradiologist’, AJNR Am J Neuroradiol, vol. 38, no. 12, pp. 2222–2230, Dec. 2017, doi: 10.3174/ajnr.A5314.
