Image Derived Input Function and Motion Correction for Kinetic Modeling of Dynamic [18F] FET-PET/MRI for Gliomas
Aim and Research Question(s)
The aim of this thesis was to enhance a pharmacokinetic protoype for 18F-FET PET/MRI for gliomas by incorparating a quantitative accurate partial volume correction method, aiming to enhance the accuracy in kinetic modeling. Alongside the partial volume correction a motino correction was adapted to address the challenge of patient movement during PET imaging. Main Hypothesis: The developed Image-Derived Input Function and motin correction can provide quantitatively accurate represenations of tracer concentrations over time for kinetic modeling, eliminating the need for invasive arterial blood sampling.
Background
Combined PET and MRI scans, using the tracer 18F-FET, allow for a detailed study of the metabolic dynamics of gliomas. For accurate diagnosis, quantifying tracer uptake is crucial. This requires measuring the input function, which indicates the cumulative availability of the radiotracer in arteries. This was done via arterial cannulation, but a non-invasive method using an IDIF is now preferred. However, this method has complications due to partial volume effects from the PET scanner's limited spatial resolution. Further patient movement during PET scans introduces artifacts and blurring, resulting in inaccurate tracer distribution. To address these issues and enhance a pharmacokinetic prototype, a partial volume correction and motion correction method were adapted. [1, 2]
Methods
Using python, a motion correction method was developed to address patient movement during PET scans, ensuring accurate alignment of Regions of Interest (ROIs) across all frames. Next a partial volume correction technique was adapted to correct for signal spillover in PET data, enhancing the accuracy of tracer concentration measurements and ensuring precise quantification in kinetic modeling. The efficacy of these corrections was assessed using the area under the curve, peak activity and the paired t-test for statistical evaluations.
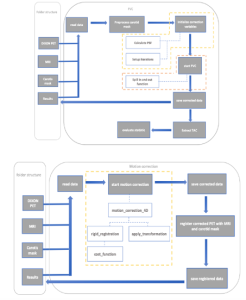
Results and Discussion
The main result of this thesis was the implementation of motion correction and a quantitatively accurate IDIF for automatic kinetic modeling of 18F-FET-PET/MRI scans. The effectiveness of motion correction was visually assessed, revealing that minor head tilts were effectively corrected. The PVC addressed tissue spillover effects, enhancing the accuracy of tracer concentration signals in the carotid artery. The corrections led to significant increases in AUC and peak activity, confirming the improved reliability of PET data and supporting the potential of non-invasive techniques for accurate kinetic modeling.
Conclusion
The findings suggest that quantitatively accurate IDIFs can replace arterial blood sampling, ensuring consistent and reproducible measurements in clinical and research settings.
References
[1] N. Upadhyay et al. (2011), [2] M. H. Poglitsch (2022)
